11+ orbital diagram for boron
Lewis is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adductA Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to. 6 to 30 characters long.

Molecular Orbital Diagram Wikiwand
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
. Khuyagbaatar and others states the superheavy element with atomic number Z 117 ununseptium was produced as an evaporation residue in the 48 Ca and 249 Bk fusion reaction at the gas-filled recoil separator TASCA at GSI Darmstadt Germany. The orbitals are p x p y and p z and each orbital can have a maximum of two electrons. NCERT TEXTBOOK QUESTIONS SOLVED.
The main group elements with an odd atomic number include the alkali metal family boron family nitrogen family and halogen family. Explain the formation of a chemical bond. Now To get the number of neutrons in a Carbon atom look at its atomic mass which is 12011 rounded to 12 and the number of protons in carbon is 6.
The term was coined and named after Serbian geophysicist and astronomer Milutin MilankovićIn the 1920s he hypothesized that variations in eccentricity axial tilt and precession combined to result in cyclical variations in the intra-annual and latitudinal. An atom of boron atomic number 5 contains five electrons. Study of WSe2WS2 moiré superlattices reveals the phase diagram of the triangular-lattice Hubbard model including a Mott insulating state at half-filling and a possible magnetic quantum phase.
The bottom-up approach of using chemical-vapour deposition produces high-quality heterostructures91011 but becomes increasingly difficult for high-order superlattices. Here m is the azimuthal quantum number of the total interatomic orbital. The ground-state electron configuration of fluorine is 1s 2 2s 2 2p 5.
A rocket engine uses stored rocket propellants as the reaction mass for forming a high-speed propulsive jet of fluid usually high-temperature gas. Boron atoms containing vacant p orbital can act as strong acceptors with p z π conjugation system 24Materials possessing an orthogonal geometry with a large. Term symbols with LS coupling.
The caesium Cs is a main group element located in period 6 of the periodic table. NCERT Solutions Class 11 Chemistry Chemistry Lab Manual Chemistry Sample Papers. Thats all this is our Bohr model of the Sodium atom that contains 11 protons and 12 neutrons in the nucleus region and 11 electrons are orbited around the nucleus two electrons in the first shell eight electrons in the second shell and one electron in the third shell.
The aldol reaction is a means of forming carboncarbon bonds in organic chemistry. Also Read-Oxygen Bohr model. Such a structure not only efficiently activates molecular oxygen and propane to promote dehydrogenation but also.
Thus the electron configuration and orbital diagram of lithium are. The sigma bonds between C and O atoms are formed by the 2sp2 orbital of C and O atoms. The fourth electron fills the remaining space in the 2s orbital.
Where X is any atom or molecule X is. The radioactive decay of evaporation residues and their α-decay products. It forms a sigma bonding and antibonding orbital.
ASCII characters only characters found on a standard US keyboard. It results in sigma bonding and antibonding orbitals. Power within an electrical circuit is only present when BOTH voltage and current are present.
Band diagram of the monolayer MoS 2 photodetector taking into consideration small. We already know that the p-subshell has three orbitals. An electron transfer mechanism that involves a light-triggered geometric conversion between metal and oxygen redox chemistry shows superior performance compared with approaches that use either.
Using molecular-beam epitaxy we synthesize heterostructures of topological insulator Bi 2 Se 3 and the Ising superconductor monolayer NbSe 2By changing the Bi 2 Se 3 thickness they demonstrate. Molecular design and synthesis. This is called quantum jump.
A Lewis acid named for the American physical chemist Gilbert N. Must contain at least 4 different symbols. This can occur in two ways.
Milankovitch cycles describe the collective effects of changes in the Earths movements on its climate over thousands of years. Atoms can jump from one orbital to another orbital in the excited state. 11 5 protons 6 number of neutrons in the atom.
An atom of the alkaline earth metal beryllium with an atomic number of 4 contains four protons in the nucleus and four electrons surrounding the nucleus. In physics and chemistry ionization energy IE American English spelling ionisation energy British English spelling is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom positive ion or molecule. According to Kossel and Lewis atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration.
MO Diagram of H2CO3. Is Cs a main group element. For example in an open-circuit condition voltage is present but there is no current flow I 0 zero therefore V0 is 0 so the power dissipated within the circuit must also be 0Likewise if we have a short-circuit condition current flow is present but there is no voltage V 0 therefore 0I 0.
On 1 May 2014 a paper published in Phys. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same. The first ionization energy is quantitatively expressed as Xg energy X g e.
The sigma bond between oxygen and hydrogen use 1s orbital of hydrogen and 2sp3 orbitals of oxygen. For light atoms the spinorbit interaction or coupling is small so that the total orbital angular momentum L and total spin S are good quantum numbersThe interaction between L and S is known as LS coupling RussellSaunders coupling named after Henry Norris Russell and Frederick Albert Saunders who described this in 1925 or spin-orbit. Herein we report a new active center with a BOHOHSi 2 structure in the borosilicate MFI framework BS-1 for this reaction that contains only isolated boron species which differs from general catalysts containing BOB oligomers.
Then to find the number of neutron round the atomic mass to the near whole number so atomic mass 10811 round to 11. These dimensions are much smaller than the diameter of the atom itself nucleus electron cloud by a factor of about 26634 uranium atomic radius is about 156 pm 156 10 12 m 9. Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872 the reaction combines two carbonyl compounds the original experiments used aldehydes to form a new β-hydroxy carbonyl compoundThese products are.
Rocket engines are reaction engines producing thrust by ejecting mass rearward in accordance with Newtons third lawMost rocket engines use the combustion of reactive chemicals to supply the necessary energy but non. The diameter of the nucleus is in the range of 170 fm 170 10 15 m for hydrogen the diameter of a single proton to about 117 fm for uranium.

Boron Orbital Diagram Electron Configuration And Valence Electrons
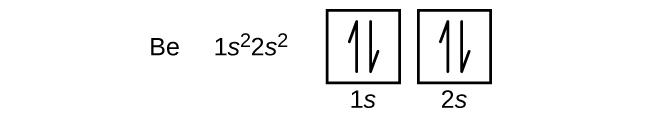
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts

Homoleptic Platinum Silver Superatoms Protected By Dithiolates Linear Assemblies Of Two And Three Centered Icosahedra Isolobal To Ne2 And I3 Journal Of The American Chemical Society

What Is The Electron Configuration Of Boron All Famous Faqs
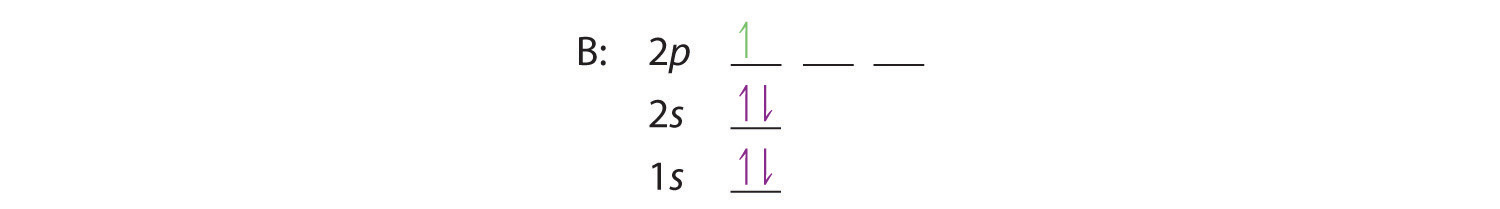
Building Up The Periodic Table

Photoelectron Spectra Of B X X 10 15 A At 193 Nm The Vertical Download Scientific Diagram

Orbital Filling Diagrams The Cavalcade O Chemistry

Nihonium Origin Uses Facts Study Com

Electron Configuration For Boron B
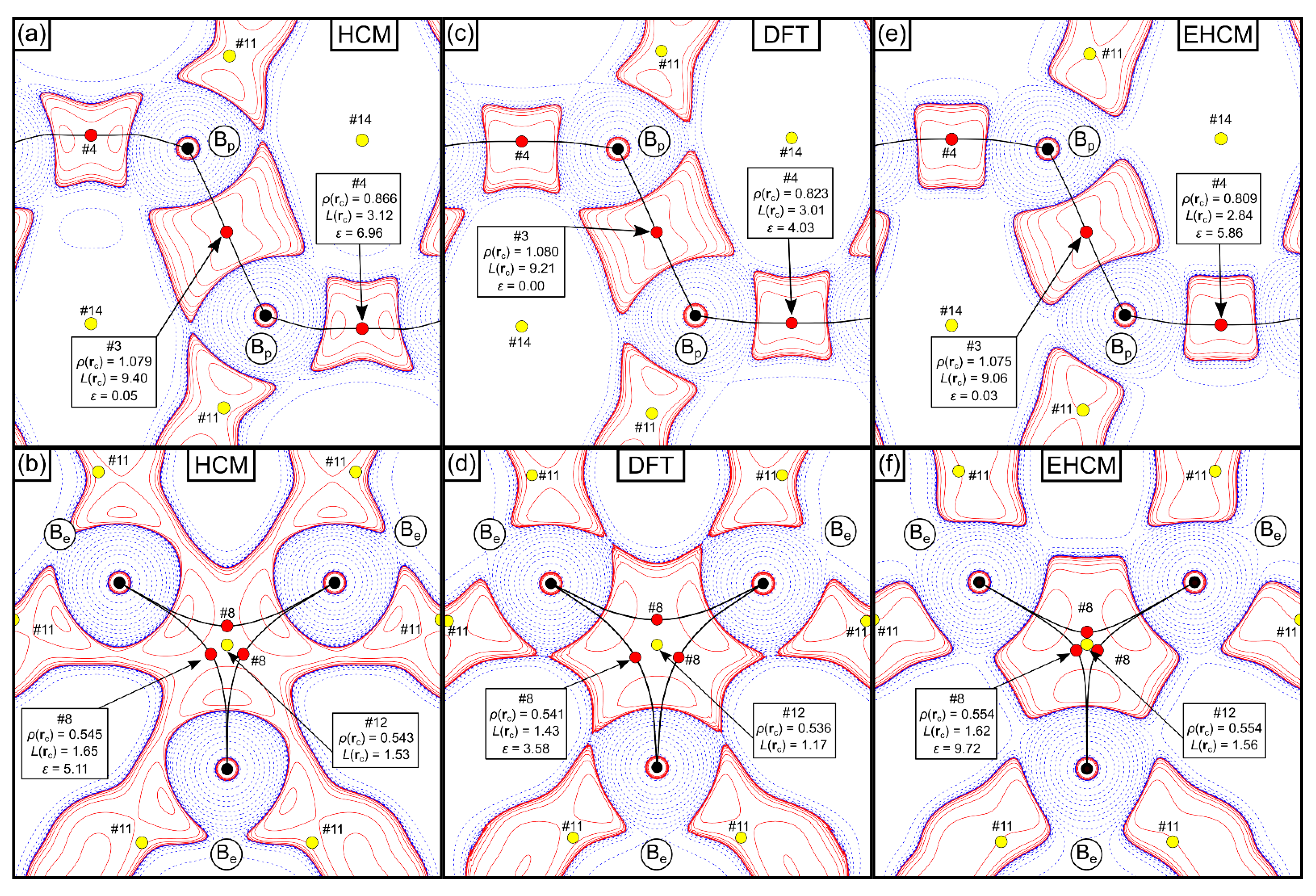
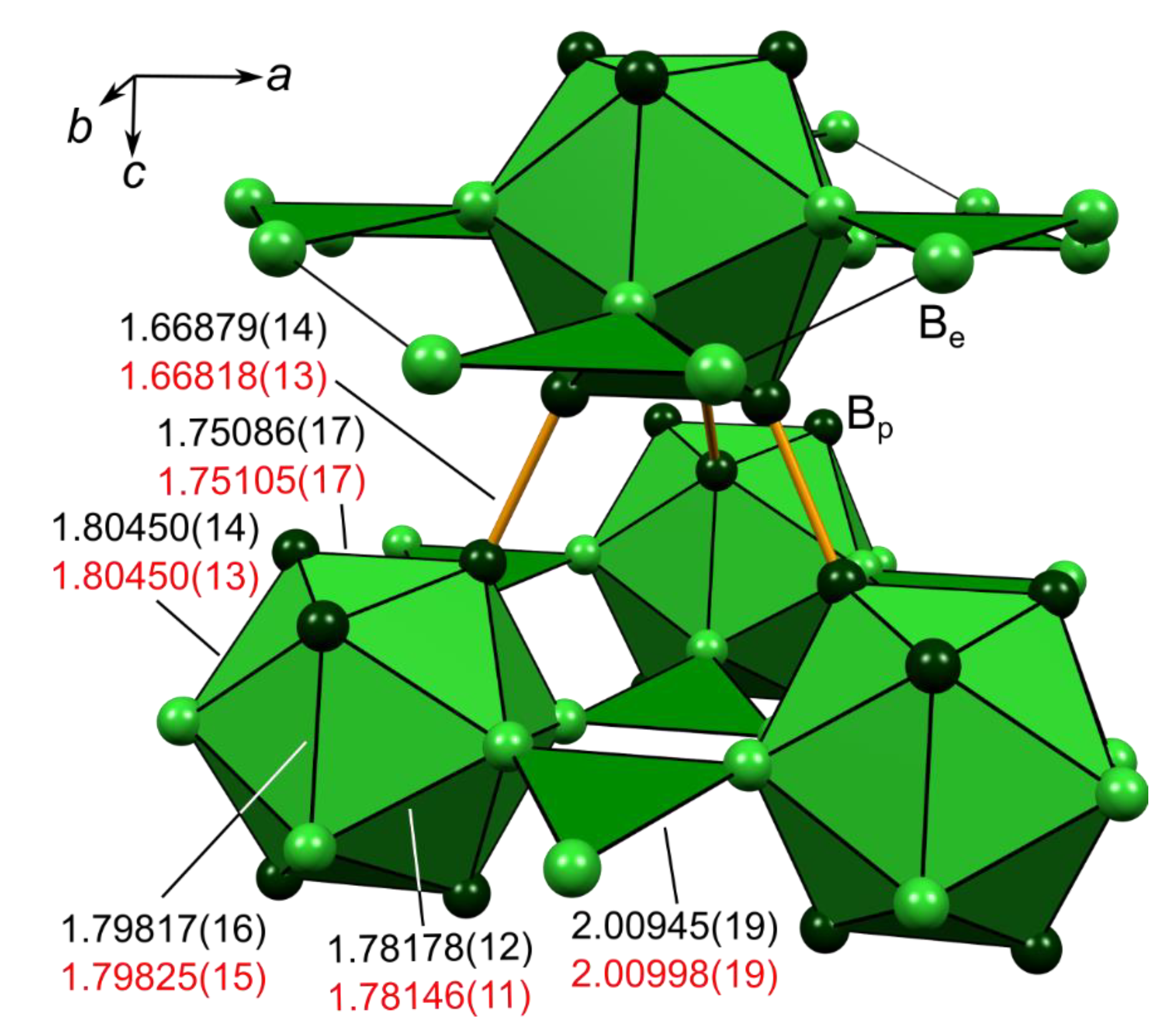
Molecules Free Full Text The Effects Of Chemical Bonding At Subatomic Resolution A Case Study On A Boron Html
Index Of Files 1210252020285154 Images
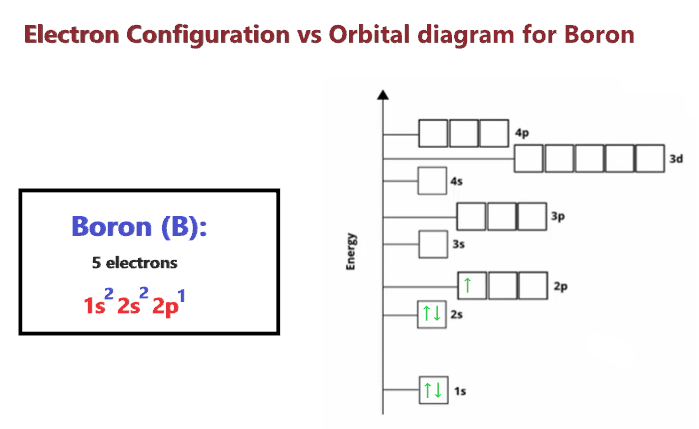
Boron Orbital Diagram Electron Configuration And Valence Electrons
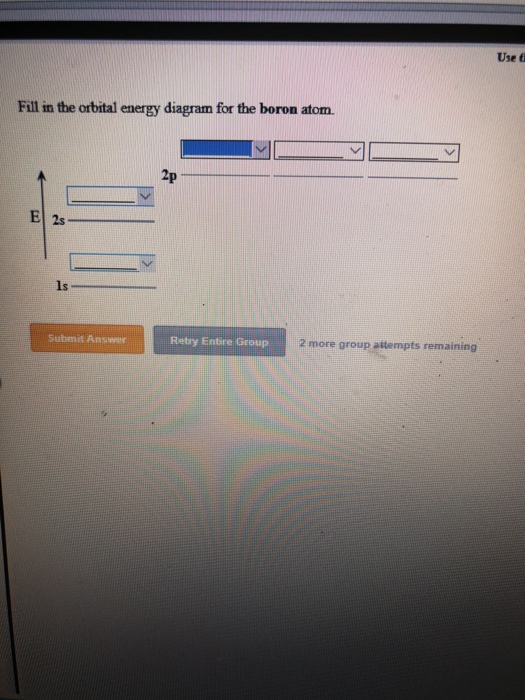
Solved Use F Fill In The Orbital Energy Diagram For The Chegg Com

Molecular Orbital Diagram Of Boron Molecule Nature Of Chemical Bond Chemistry Class 11 Youtube

Boron B

How To Write The Orbital Diagram For Boron B Youtube

Molecular Orbital Diagram Of Boron Molecule Nature Of Chemical Bond Chemistry Class 11 Youtube